Antibody-drug conjugates (ADCs) have become a very promising therapeutic modality for antibody drug research and development, due to their ability to deliver a cytotoxic payload in an antigen-specific manner. Through decades of development, ADC technologies- including coupling and linker/payload- have made great breakthroughs. However, because the currently approved targets of ADCs are very limited, identifying safe and effective innovative targets remain the key to the future success of ADC research and development.
Mechanistically, bispecific antibodies can induce effector mechanisms not possible with monospecific antibodies or combination antibody therapy. Thus, bispecific ADCs targeting dual tumor-associated antigens (TAAs) have multiple potential anti-tumor advantages:
1. Simultaneous targeting of dual tumor-driven signaling pathways to overcome drug resistance; 2. Improved specificity for tumor cells co-expressing dual TAAs to reduce off-target toxicity; and 3. Imparting a new synergistic mechanism of dual-target binding and endocytosis, resulting in a more efficient tumor killing.
Overview of Biocytogen’s Bispecific ADC platform
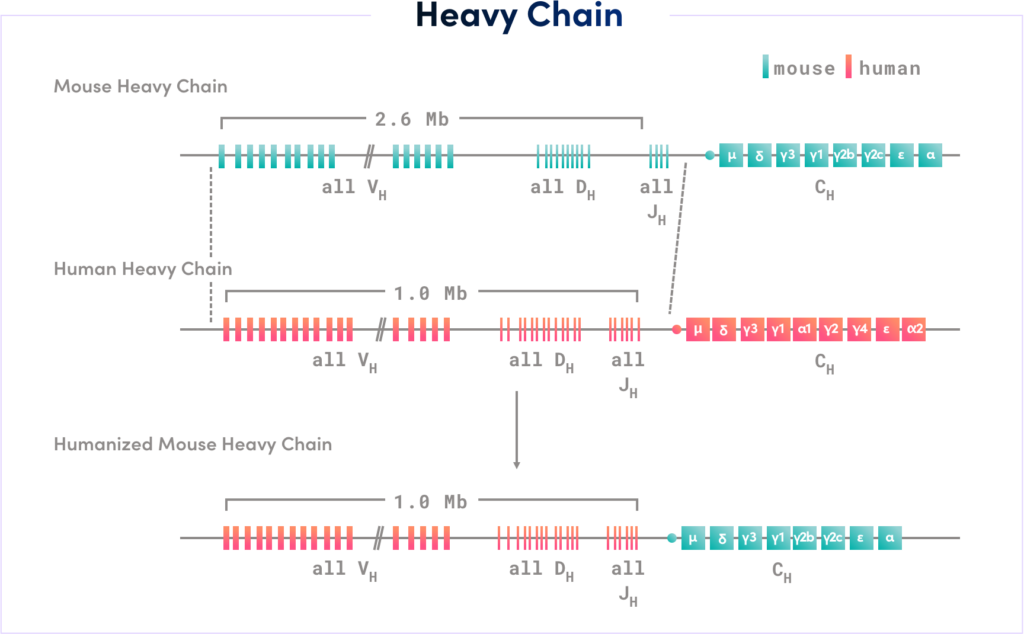
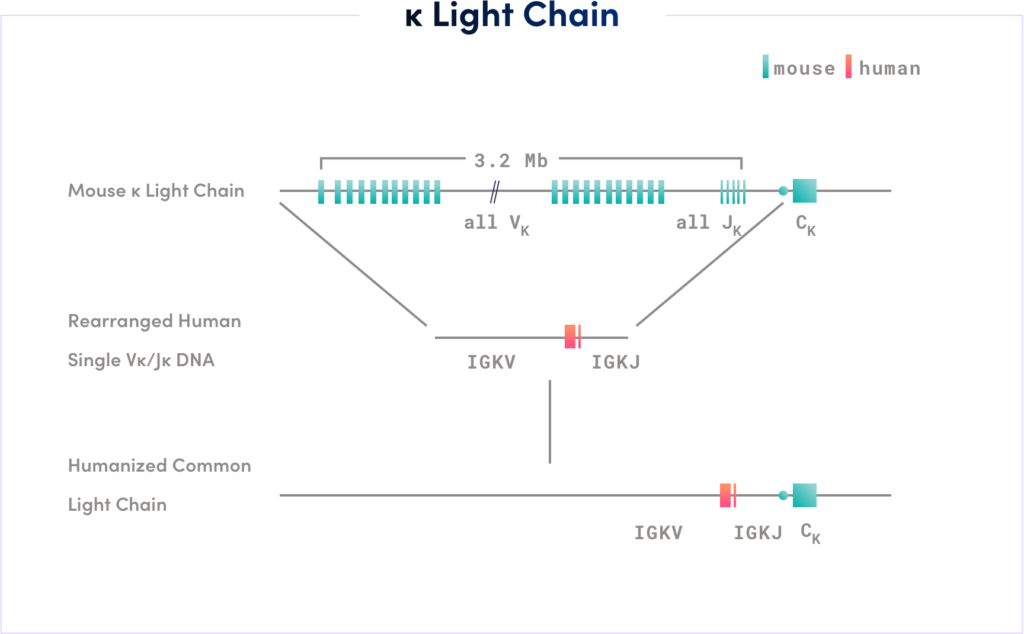
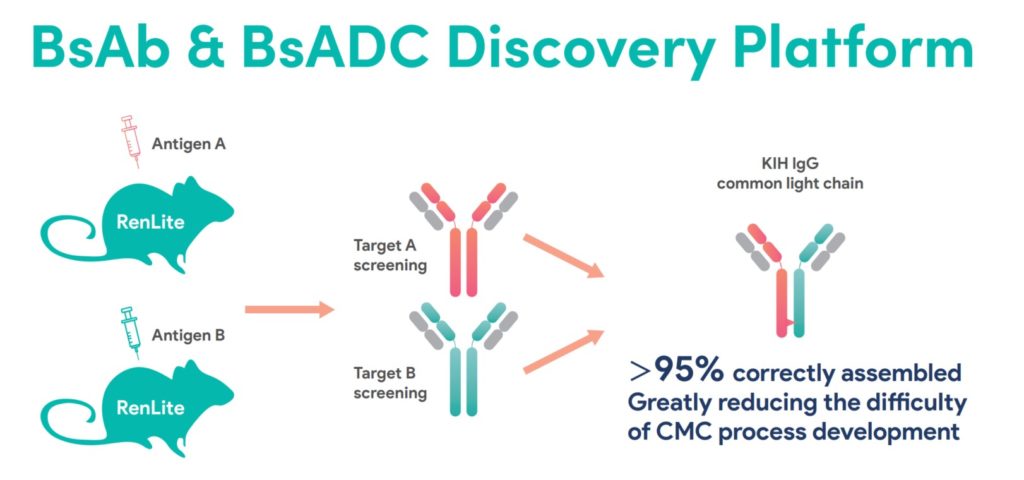
The fully human antibodies produced by RenLite® mice have a common kappa light chain, which can effectively solve the mismatch problem between heavy chain and light chain during the assembly of BsAbs, facilitating drug development.
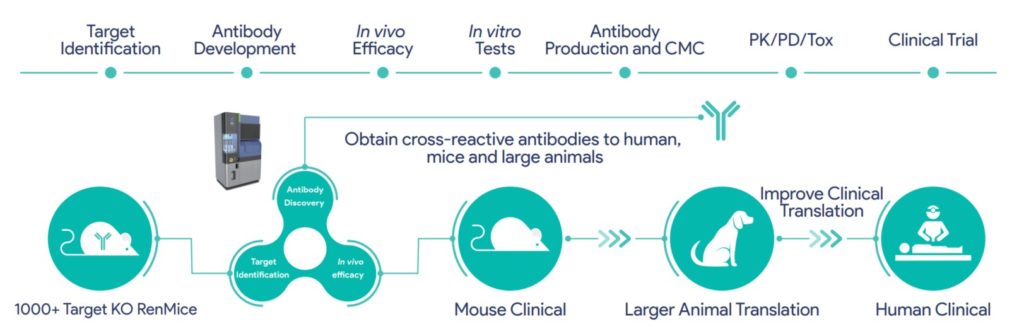
RenLite® mice can produce fully human antibodies with diverse epitopes and high affinity. In addition, KIH (knobs-into-holes) technology is used to connect the heavy chains of the two parental monoclonal antibodies, ensuring the successful assembly of a BsAb molecule with a stable monoclonal antibody structure. Combinations of TAA-targeting antibodies generated from RenLite mice are subsequently screened in a high-throughput manner.
As part of Biocytogen’s Project Integrum, bsADCs assembled from TAA-targeting antibodies will be further evaluated by in vivo pharmacologic efficacy and in vitro analyses. The company’s large animal translational platform will further screen out products with better translational properties:
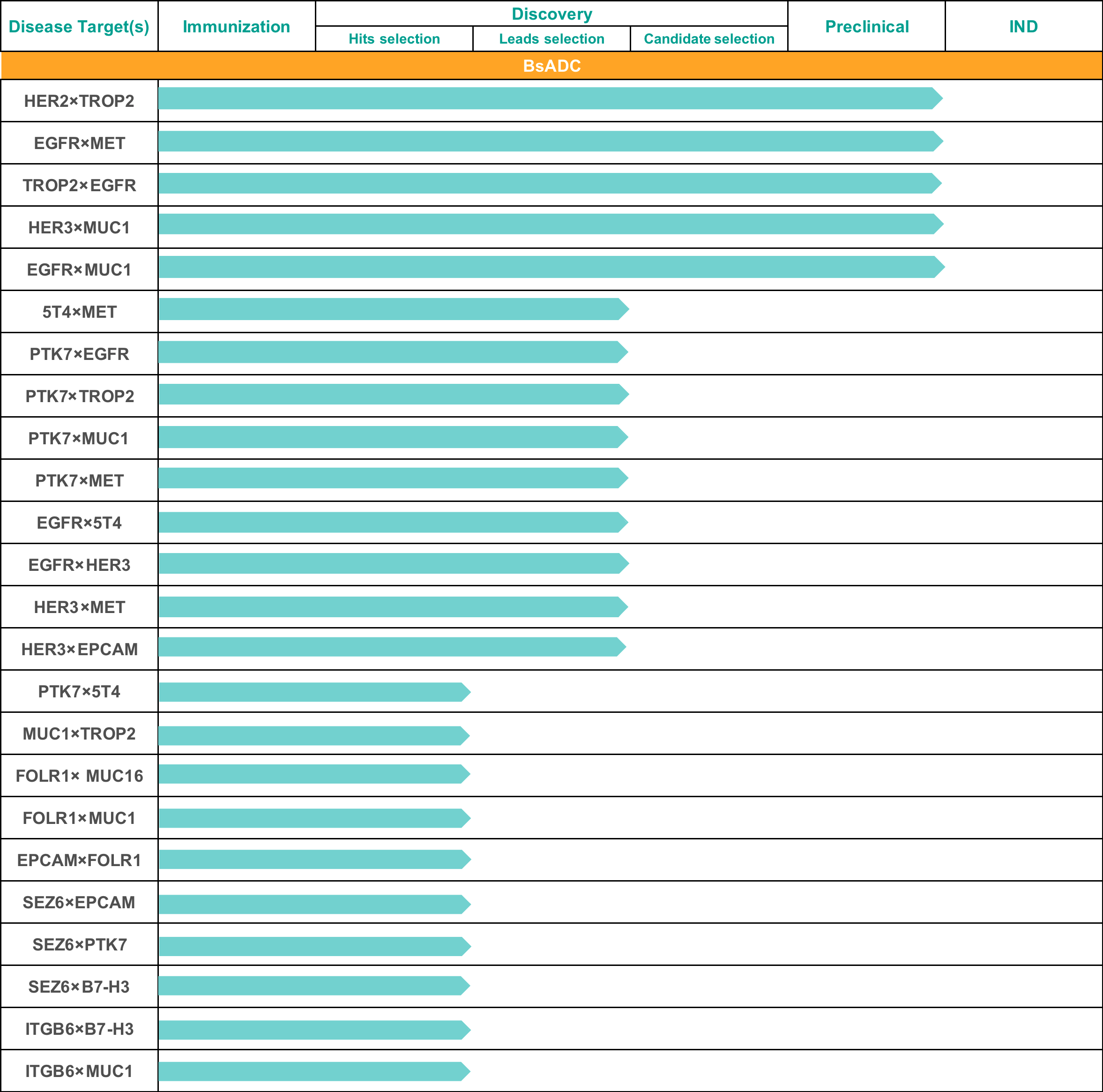
Bispecific ADC Development Progress
Poster
AACR 2023: BCG022: A Novel Bispecific Antibody-Drug Conjugate Targeting HER3 and MET
AACR 2023: Identification of DM004, A First-In-Class Anti-5T4/MET Bispecific Antibody-Drug Conjugate