In Vivo Pharmacology Services
In vivo efficacy studies
Biocytogen provides high-quality in vivo pharmacology services focused on efficacy evaluation of novel therapeutics. Our in vivo efficacy studies utilize genetically modified mouse models expressing human targets knocked into the loci of their respective mouse counterparts, severely immunodeficient (B-NDG) mice and their variants, as well as wild-type mouse models to support drug discovery and development in the areas of oncology, immuno-oncology, autoimmune diseases, and other inflammatory diseases.
Our experts have the capability to test the in vivo efficacy of a wide range of biologics including mono- and bispecific antibodies, vaccines, blood components, allergenics, gene therapy, cells/tissues, and recombinant therapeutic proteins. Some brief examples are provided directly below; for more in-depth information about these in vivo pharmacology services, please visit these pages:
- Syngeneic Tumor Models for Antibody Screening
- CAR-T efficacy evaluation
- Anti-Tumor Efficacy in CDX & Humanized Models
Example Studies
-
Antibody Efficacy Study in Immune-competent Syngeneic Mouse Models
-
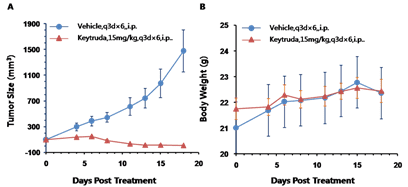
Murine colon cancer MC38 cells were subcutaneously implanted into homozygous humanized B-hPD-1 mice, where the extracellular domain of human PD-1 replaces that of mouse PD-1 via genomic knock-in. Mice were grouped when the tumor size reached 150 ± 50 mm3 (n=10). The human anti-PD-1 antibody (red line) significantly inhibited tumor growth, confirming that the B-hPD-1 mouse model is a powerful tool for in vivo efficacy studies. Tumor volume: mean ± SEM (A). Body weight: mean ± SEM (B).
-
Bispecific Antibody Efficacy Study
-
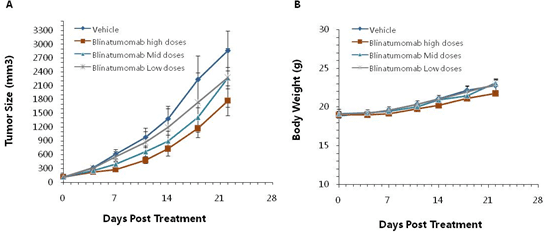
Murine colon cancer cells MC38 engineered to express human CD19, MC38-hCD19, were subcutaneously implanted into B-hCD3e mice, where the extracellular domain of human CD3e replaces that of mouse CD3e via genomic knock-in. Mice were grouped (n=6) when the tumor sizes were approximately 150 ± 50 mm3. Blinatumomab, a bispecific antibody targeting human CD3e and human CD19, significantly inhibited tumor growth, demonstrating that the B-hCD3e mice are a powerful model for in vivo efficacy evaluation of anti-hCD3e-based bispecific antibodies. Tumor volume: mean ± SEM (A). Body weight: mean ± SEM (B).
-
Small Molecule Efficacy Study
-
Small molecules are an important anti-cancer therapeutic modality, either as single agents or in combination with antibody therapeutics. Biocytogen has extensive experience in testing small molecule therapeutics, as illustrated in the following example.
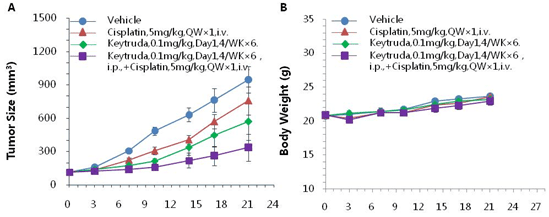
Murine colon cancer MC38-hPD-L1 cells were subcutaneously implanted into homozygous B-hPD-1 mice. Mice were grouped when the tumor size reached approximately 150 ± 50 mm3 (n=8). Combination of anti-hPD-1 antibody and the chemotherapy drug cisplatin (purple) showed additional inhibitory effects on tumor growth compared with individual agents alone in humanized B-hPD-1 mice. Tumor volume: mean ± SEM (A). Body weight: mean ± SEM (B).
-
CAR-T Cell Efficacy Study in Immunodeficient B-NDG Mice
-
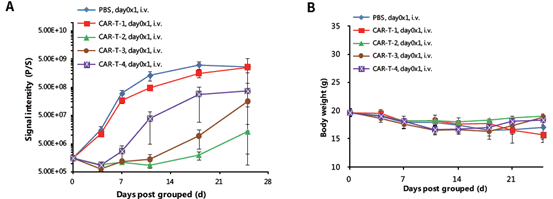
Efficacy of various CAR-T therapy targeting human CD20 was evaluated in highly immunodeficient B-NDG mice inoculated with modified human B cell lymphoma cells, B-Raji-Luc-GFP. Various CAR-T cells were injected i.v. at the same time of B-Raji-Luc-GFP cells. (A) Luciferase signals as an indicator of tumor load in mice treated with different CAR-T cells. (B) Body weight of treated mice. All values are expressed as mean ± SEM.