Experimental Autoimmune Encephalomyelitis (EAE) Model
Experimental Autoimmune Encephalomyelitis (EAE) is a relevant preclinical model of multiple sclerosis (MS). MS is a central nervous system chronic inflammatory disease which leads to brain inflammation and demyelination. MS is considered an autoimmune disorder caused by auto-reactive T cells with symptoms including muscle stiffness and paralysis, visual disturbances and blindness, sensory loss, and ataxia. The disease is prone to relapse and remission. There are several different animal models of MS. The EAE model is widely used because the pathological features of inflammation and demyelination induced in EAE is similar to that observed in MS disease. Among factors that influence the disease, the IL-23/IL-17 axis has been implicated in the pathogenesis of EAE and MS1-3.
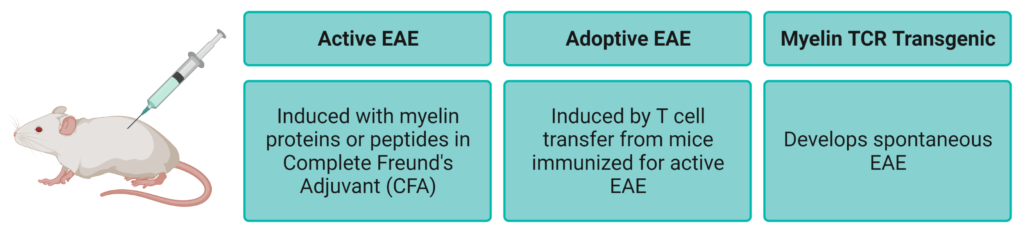
The EAE model of multiple sclerosis can be induced by immunization with the proteins derived from the myelin sheath, such as myelin-oligodendrocyte glycoprotein (MOG), and Complete Freund’s Adjuvant (CFA) accompanied by an intraperitoneal injection of pertussis toxin (PTX) on the day of immunization and two days later. Myelin-specific T cells are activated in the periphery, migrate through the blood-brain barrier into the CNS and are reactivated, triggering a series of inflammatory reactions that lead to demyelination and axon cell death, and ultimately to nerve injury and disability.
Biocytogen provides a robust MOG-induced EAE model protocol for efficacy studies. Clinical symptoms are assessed using a standard scoring system that measures the degree of disease induction. Local demyelination and Inflammatory leukocyte infiltration can be visualized by histopathological staining. Furthermore, we can induce EAE in B-hIL17A mice, where the human IL-17A gene is knocked into the mouse IL-17A gene, thus providing a genetically humanized mouse EAE model of multiple sclerosis for convenient testing of novel therapeutics targeting human IL-17A.
Watch our December 2021 webinar: Investigating Inflammatory Disease using Humanized Cytokine Mice