AACR 2022: A Humanized Mouse Model of the Promising Immune Checkpoint Molecule B7-H4
Conclusions
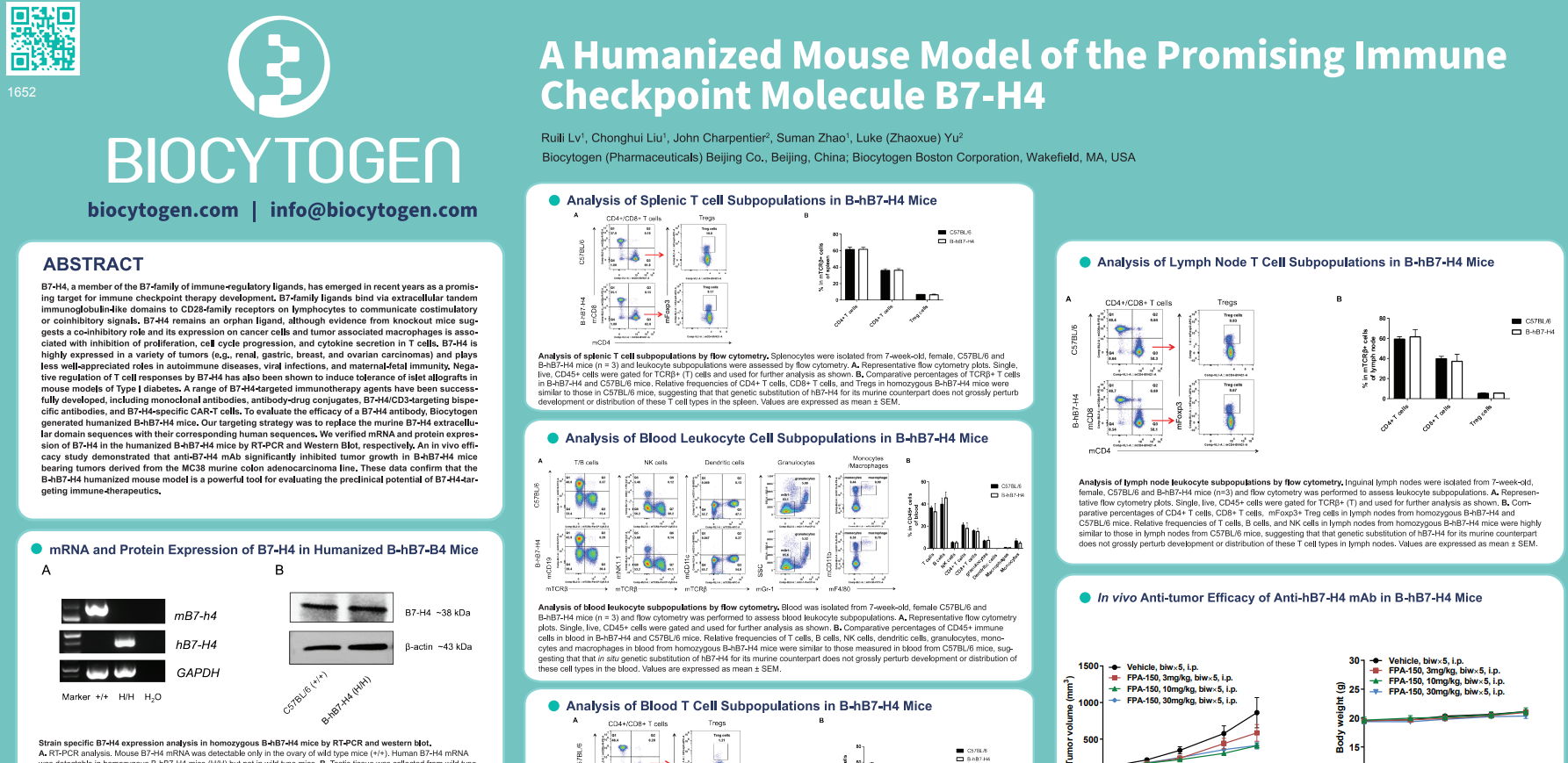
- As a negative regulator of T cell responses that is highly expressed in a range of carcinoma types, B7-H4 has emerged in recent years as a promising target for immune checkpoint therapy development. We sought to develop a humanized B7-H4 mouse model (B-hB7-H4) by in situ substitution of the murine extracellular domain sequences with their corresponding human sequences.
- After verifying mRNA and protein expression by RT-PCR and Western blot respectively, we profiled and compared immune cell populations in the homozygous B-hB7-H4 mice and wild type mice. Immune cell
composition and frequencies in homozygous B-hB7-H4 mice are comparable to those observed in the
parental C57BL/6 strain. Prior evidence from knockout mice and cell line studies suggested a co-inhibitory function for B7-H4, namely via inhibition of proliferation, cell cycle progression, and cytokine secretion in T cells. - An ongoing in vivo efficacy study demonstrated that anti-hB7-H4 antibodies significantly inhibited tumor growth in B-hB7-H4 mice bearing tumors derived from the MC38 (murine colon adenocarcinoma) line.
- Together, these data confirm that the B-hB7-H4 humanized mouse model is a powerful tool for evaluating the preclinical potential of B7-H4-targeting immune therapeutics such as monoclonal antibodies, antibody-drug conjugates, and CAR T cells.