Our innovative gene editing service technology increases gene-editing efficiency by 10 to 20-fold, making our custom model development process faster and more affordable for your research.
| Technology | Advantages & Disadvantages | Applications | Timeline | ||||||
| ESC/HR-based Gene Editing |
|
| 7-11 months |
| Technology | Advantages & Disadvantages | Applications | Timeline | ||||||||
CRISPR/EGE™-based Gene Editing |
|
| 5-7 months |
| Technology | Advantages & Disadvantages | Applications | Timeline | ||||||
ESC/HR-based Gene Editing |
|
| 7-11 months |
| Technology | Advantages & Disadvantages | Applications | Timeline |
Random genetic insertion (Tol2-Tg, BAC-Tg) | Fast timeline Low cost | Transgenic mice/rats | 2-3 months for F0 |
| Technology | Advantages & Disadvantages | Applications | Timeline |
Chromosome Engineering |
| Mouse | Upon request |
| Technology | Advantages & Disadvantages | Applications | Timeline |
Other Services |
| CRISPR plasmid construction; CRISPR activity assay; Donor plasmid construction | Upon request |
Genetically modified animal models are invaluable tools for studying gene function under physiological and disease conditions. Generating these models can utilize transgenic technology or site-specific gene editing, and the strategy used depends on the size of the modification as well as the desired readout. For the introduction of large genomic fragments, Bacterial artificial chromosome (BAC) transgenic technology or Tol2-mediated integration randomly are suitable choices to integrate foreign DNA into the target genome. Conversely, site-specific gene modification usually employs homologous recombination to introduce exogenous DNA. Homologous recombination is a natural genetic recombination event in which specific nucleotide sequences are randomly exchanged between similar or identical residues.
We as scientists have learned to utilize the natural phenomena of homologous recombination to genetically engineer mouse embryonic stem cells (ESC) in a site-specific manner. Gene targeting technology has significantly evolved in recent years, with the emergence of DNA nuclease-based gene-editing, including Zinc Finger Nuclease (ZFN), and more recently, CRISPR/Cas9 technology, which allows even more precise and intentional genetic changes. Compared to ZFN and TALEN, CRISPR/Cas9 technology is more efficient, shortens the timeline/reduces the cost of production, eliminates species constraints, and has the potential to edit genes in patient tissues directly.
Historically, the efficiency of such modifications is limited due to the low rates of homologous recombination. To overcome this challenge, Biocytogen developed an innovative gene targeting technology: the CRISPR/Cas9-based Extreme Genome Editing — EGE™ — system, which can knock in large DNA fragments (> 5 kb) in the genomes of mice, rats, and cell lines up to 20-fold more efficiently than conventional CRISPR/Cas9 methods. EGE™ is successful for 98% of projects, which accelerates the timeline to generate genetically engineered animal and cell models.
Biocytogen incorporates a bioinformatics approach to minimize off-target activity by searching the target genome for regions of similar sequence identity to the sgRNAs. Therefore, only sgRNAs with high specificity and high activity are chosen. As part of our quality control measures, we can perform 2 rounds of PCR to amplify the genomic regions around potential off-target sites, followed by sequencing to look for off-target events. We have also integrated Southern blot in our workflow as an important quality control step for generating knock-in and conditional knockout animal models to detect any potential random insertions. A dedicated project manager will be assigned to your project once it is initiated, they will provide monthly updates on the status of your model and are available to be reached at any time.

Check out our FAQ page for more information.
For more detailed information about our gene editing service technologies, Download Gene Editing Brochure.
Aim: Flvcr2 Conditional Knock-Out (cKO) and Reporter Knock-in (KI) by FLEx Strategy
Publication:
Lack of Flvcr2 impairs brain angiogenesis without affecting the blood-brain barrier
Santander N, Lizama CO, Meky E, McKinsey GL, Jung B, Sheppard D, Betsholtz C, and Arnold TD (2020) Lack of Flvcr2 impairs brain angiogenesis without affecting the blood-brain barrier. J Clin Invest, 130, 4055-4068.
Aim: Flvcr2 Conditional Knock-Out (cKO) and Reporter Knock-in (KI) by FLEx Strategy
Publication:
Lack of Flvcr2 impairs brain angiogenesis without affecting the blood-brain barrier
Santander N, Lizama CO, Meky E, McKinsey GL, Jung B, Sheppard D, Betsholtz C, and Arnold TD (2020) Lack of Flvcr2 impairs brain angiogenesis without affecting the blood-brain barrier. J Clin Invest, 130, 4055-4068.
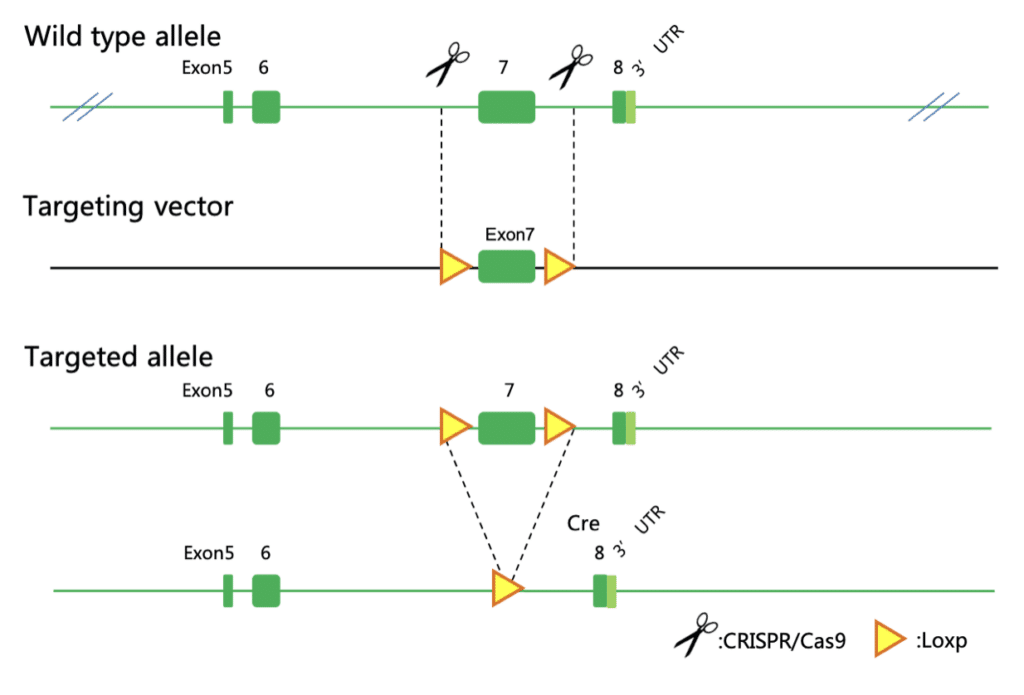
Aim: Microglial P2y12 Receptor Conditional Knock-Out (cKO) by CRISPR/Cas9-based Extreme Genome Editing (EGETM) System
· Allele Design & Validation: Guide RNAs (gRNAs) were designed to target the regions upstream of exon 4 and downstream of the 3’UTR of P2ry12. Exon 4 was flanked with one loxP fragment inserted into intron 3 and the other was inserted downstream of the 3’UTR. P2Y12-floxed mice were then crossed with the CX3CR1Cre/+ mice to obtain P2Y12f/f;CX3CR1Cre/+ (conditional KO) mice. Upon Cre-mediated recombination, exon 4 was removed, leading to the inactivation of the gene. Immunostaining results showed that P2Y12R expression was completely removed from the conditional KO mice in the brain of adult mice.
· Biologic Results: P2y12 receptor deficiency increased seizure severity in P2Y12f/f;CX3CR1Cre/+ mice compared to WT mice. The results from microglial P2y12 receptor conditional knock-out mice indicate that microglia enhance epileptogenesis and targeting microglia in general or microglial P2Y12R in specific to ameliorate proepileptogenic processes could be a novel therapeutic strategy in the clinic.
Publication:
Microglial P2Y12 receptor regulates ventral hippocampal CA1 neuronal excitability and innate fear in mice
Peng J, Liu Y, Umpierre AD, Xie M, Tian DS, Richardson JR, and Wu LJ (2019) Microglial P2Y12 receptor regulates ventral hippocampal CA1 neuronal excitability and innate fear in mice. Mol Brain, 12, 71.
Aim: hCD63-GFP Conditional Knock-in (cKI) in Rosa26 ‘Safe Harbor’ Locus
· Allele Design & Validation: hCD63-GFPf/f knock-in mice were generated by homologous recombination in embryonic stem cells. A CAG promoter, loxP-floxed stop codon, and human CD63-copGFP-6xHis cassette were inserted into the Rosa26 ‘safe harbor’ locus. The hCD63-GFPf/f mice were crossed with tamoxifen-inducible Cre mice under the control of the CaMKII promoter to generate the CaMKII-CreERTCD63-GFPf/+ mice. After 4-hydroxytamoxifen (4-OHT) injection and Cre-mediated recombination, the stop codon was removed, leading to the expression of hCD63 with a GFP tag under the control of the CAG promoter.
· Model Validation: AAV8-CaMKII-Cre_mCherry virus particles from which mCherry-tagged Cre is expressed under the CaMKII promoter were focally injected into the motor cortex of CD63-GFPf/+ mice. Both CD63-GFP and mCherry signals are observed in the proximal section of the injection site.
· Biologic Results: The Rosa26-hCD63-GFP conditional knock-in mice demonstrated that neuronal exosomes can be secreted and transferred into astrocytes in vivo. This finding potentially opens a new frontier in understanding CNS intercellular communication.
Publication:
Exosome reporter mice reveal the involvement of exosomes in mediating neuron to astroglia communication in the CNS
Men Y, Yelick J, Jin S, Tian Y, Chiang MSR, Higashimori H, Brown E, Jarvis R, and Yang Y (2019) Exosome reporter mice reveal the involvement of exosomes in mediating neuron to astroglia communication in the CNS. Nat Commun, 10, 4136.
B-hTFR1 mice
Aim: Generation of a humanized TFR1 mouse model
Biologic Results: Anti-human TFR1 bispecific antibody was successfully detected in brain tissues of the B-hTFR1 mouse after intravenous administration of the antibody, suggesting a robust uptake of the antibody via the hTFR1 receptor.
Figure 1. Targeting strategy for the generation of humanized TFR1 mice.
View our extensive list of publications featuring our gene editing services and off-the-shelf models.
